Business
Coronavirus vaccine from Pfizer and BioNTech is strongly effective, early data from enormous trial indicate
Published
5 years agoon
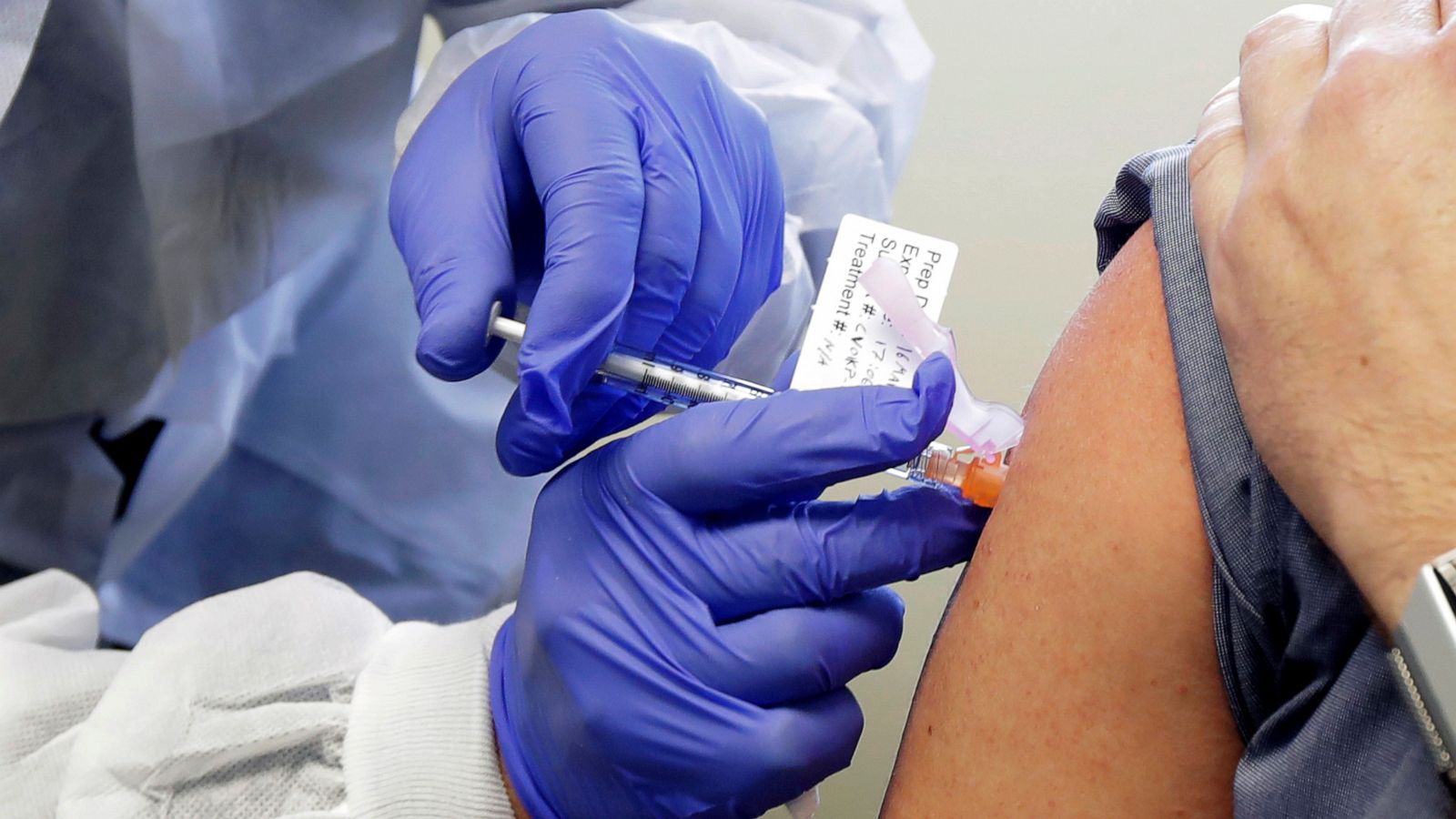
Pfizer and accomplice BioNTech said Monday that their immunization against Covid-19 was firmly powerful, surpassing desires with results that are probably going to be met with wary energy — and help — despite the worldwide pandemic.
The antibody is the first to be tried in the United States to produce late-organize information. The organizations said an early examination of the outcomes demonstrated that people who got two infusions of the immunization three weeks separated experienced over 90% less instances of indicative Covid-19 than the individuals who got a fake treatment. For quite a long time, specialists have forewarned that an antibody that may just be 60% or 70% powerful.
The Phase 3 investigation is progressing and extra information could influence results.
With regards to direction from the Food and Drug Administration, the organizations won’t petition for a crisis use approval to circulate the antibody until they arrive at another achievement: when half of the patients in their investigation have been noticed for any wellbeing issues for in any event two months following their subsequent portion. Pfizer hopes to pass that boundary in the third seven day stretch of November.
“I’ve been in vaccine development for 35 years,” William Gruber, Pfizer’s senior vice president of vaccine clinical research and development, told STAT. “I’ve seen some really good things. This is extraordinary.” He later added: “This really bodes well for us being able to get a handle on the epidemic and get us out of this situation.”
Despite the fact that it is a brilliant spot in the fight against the pandemic and a victory for Pfizer and BioNTech, a German organization, key data about the antibody isn’t yet accessible. There is no data yet on whether the immunization forestalls serious cases, the sort that can cause hospitalization and demise.
Nor is there any data yet on whether it keeps individuals from conveying the infection that causes Covid-19, SARS-CoV-2, without side effects.
Without more data, it’s too soon to begin anticipating the amount of an effect the antibody could make, said Michael Osterholm, head of the University of Minnesota’s Center for Infectious Diseases Research and Policy.
“I don’t want to dampen any enthusiasm for this vaccine. I just want us to be realistic,” Osterholm said. “For a vaccine to really have maximal impact, it’s going to have to also reduce severe illness and death. And we just don’t know yet.”
Since the immunization has been read for just only months, it is difficult to state how long it will ensure against contamination with the infection. The antibody causes results, including hurts and fevers, as indicated by recently distributed information. Gruber said that he accepted the result profile was practically identical to standard grown-up antibodies, yet most likely more regrettable than Pfizer’s pneumonia immunization, Prevnar, or an influenza shot.
The outcomes have not been peer-investigated by outside researchers or distributed in a clinical diary, and even Pfizer and BioNTech have been given no different insights concerning how the immunization performed by the autonomous screens directing the examination.
Beginning supplies of the immunization, whenever approved, will be restricted. Pfizer says up to 50 million dosages could be accessible worldwide. before the year’s over, with 1.3 billion accessible in 2021. There are likewise expected to be dissemination challenges. The antibody must be put away at super-chilly temperatures, which could make it amazingly hard to convey to numerous spots. Pfizer has said it is certain those issues can be overseen.
Despite the fact that the gauge of the viability of the immunization could change as the examination is finished, it is near a most ideal situation. That likewise looks good for different antibodies in the late phases of testing, including those created by Moderna, AstraZeneca, and Johnson and Johnson.
“If that headline really number really holds up, that is huge. That is much better than I was expecting and it will make a huge difference,” said Ashish Jha, the dignitary of the School of Public Health at Brown University. He advised, notwithstanding, that it is consistently hard to assess science through public statement and that analysts should see the full outcomes. He noticed that results are something to watch, on the grounds that regardless of whether there are no genuine long haul entanglements, individuals feeling wiped out for a day or two could lead some to be reluctant to take an immunization.
Both Pfizer’s immunization and Moderna’s utilization courier RNA, or mRNA, innovation, which utilizes hereditary material to make the body make a protein from the infection; the invulnerable framework at that point perceives the infection and figures out how to assault. Different antibodies in the late phases of advancement utilize hereditarily designed infections for a comparable reason, or bits of protein that are straightforwardly infused. No mRNA item has ever been affirmed by controllers.
The tale of how the information have been investigated appears to incorporate no modest quantity of show. Pfizer, seeing an occasion to both assistance fight a pandemic and show its exploration ability, settled on choices that were in every case liable to make its examination the first of a Covid-19 immunization to create information — including its choice to have a free gathering of specialists, known as an information security and checking board, investigate the information in the 44,000-volunteer examination before its finishing.
The main investigation was to happen after 32 volunteers — both the individuals who got the antibody and those on fake treatment — had contracted Covid-19. In the event that less than six volunteers in the gathering who got the antibody had created Covid-19, the organizations would make a declaration that the immunization had all the earmarks of being powerful. The investigation would proceed until in any event 164 instances of Covid-19 — people with at any rate one side effect and a positive test outcome — had been accounted for.
That review configuration, just as those of other medication producers, experienced harsh criticism from specialists who stressed that, regardless of whether it was measurably substantial, these between time examinations would not give enough information when an immunization could be given to billions of individuals.
In their declaration of the outcomes, Pfizer and BioNTech uncovered an astonishment. The organizations said they had chosen not to lead the 32-case investigation “after a discussion with the FDA.” Instead, they intended to direct the examination after 62 cases. However, when the arrangement had been formalized, there had been 94 instances of Covid-19 in the investigation. It’s not known the number of were in the immunization arm, yet it would need to be nine or less.
Gruber said that Pfizer and BioNTech had chosen in late October that they needed to drop the 32-case break examination. Around then, the organizations chose to quit having their lab affirm instances of Covid-19 in the examination, rather leaving tests away. The FDA knew about this choice. Conversations between the office and the organizations closed, and testing started this previous Wednesday. At the point when the examples were tried, there were 94 instances of Covid in the preliminary. The DSMB met on Sunday.
This implies that the factual quality of the outcome is likely far more grounded than was at first anticipated. It likewise implies that if Pfizer had held to the first arrangement, the information would almost certainly have been accessible in October, as its CEO, Albert Bourla, had at first anticipated.
Gruber said that there won’t be another interval examination directed in the investigation. He additionally said that Pfizer’s gauge that it could petition for approval of the immunization by the third seven day stretch of November depended on the suspicion that the FDA would acknowledge two-month wellbeing information on a large portion of the volunteers in the examination as at first arranged, when it was to incorporate 30,000 volunteers, not more than 44,000, as is presently the situation. Those conversations are progressing.
In any case, Gruber said he currently expects that when of the arranged gathering of the FDA’s immunization warning board of trustees in December, the examination’s adequacy bit could be finished, having arrived at 164 instances of Covid-19.
He additionally underscored that despite the fact that there may be a couple of long stretches of information from this examination, results from prior investigations make him idealistic that insusceptibility from the immunization won’t disappear quickly.
The investigation has enlisted 43,538 volunteers the organizations stated, and 38,955 have gotten their subsequent portion. About 42% of worldwide members and 30% of U.S. members have racially and ethnically assorted foundations.
Bourla, Pfizer’s CEO, said the results mark “a great day for science and humanity,” in a statement, saying they provide “initial evidence of our vaccine’s ability to prevent Covid-19.” He added: “We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming weeks.”
You may like
-
CDC information recommend Pfizer antibody insurance holds up in kids 5-11, bringing up issues on prior study
-
Study recommends antibodies from two Moderna vaccine doses less compelling at killing omicron
-
Omicron shows up more impervious to Covid antibodies yet is causing less extreme disease in South Africa, significant review discovers
-

According to CDC report ,Most revealed U.S. Omicron cases have hit the completely inoculated
-


Explainer: Omicron versus COVID-19 antibodies: What more do we have to perceive?
-
According To UK Research ,Blending Pfizer, AstraZ COVID-19 shots with Moderna gives superior immune reaction
Business
AI Startup Anthropic Nears $3.5 Billion Funding Round
Published
3 months agoon
February 25, 2025
Anthropic, a leading AI startup, is on the verge of securing $3.5 billion in a fresh funding round that could push its valuation to $61.5 billion, according to sources familiar with the matter.
The company, known for developing the Claude chatbot, is reportedly attracting investments from major venture capital firms, including Lightspeed Venture Partners, General Catalyst, and Bessemer Venture Partners.
This funding round surpasses the $2 billion target Anthropic was previously reported to be seeking last month. The surge in investor interest highlights the growing appeal of AI-driven enterprises, with nearly half of all U.S. venture funding in 2023 flowing into artificial intelligence startups.
The strong financial backing also emphasizes the dominance of U.S. AI firms, even as Chinese alternatives like DeepSeek emerge with cost-efficient models that have caught the attention of some investors.
Meanwhile, OpenAI—the company behind ChatGPT—is also in discussions for a substantial funding round that could elevate its valuation to as much as $300 billion, as reported by Reuters.
Anthropic, co-founded by former OpenAI executives Dario and Daniela Amodei, had previously achieved a valuation of around $18 billion following a funding round led by Menlo Ventures last year.
In addition to its fundraising efforts, the company recently introduced Claude 3.7 Sonnet, an advanced AI model designed to enhance response speed and improve step-by-step reasoning. This move aims to strengthen Anthropic’s position in the competitive generative AI landscape.
The funding round was first reported by the Wall Street Journal, while Anthropic has yet to comment on the ongoing investment discussions.
Business
Level Up Your Digital Game with DigiRoads – Training, Strategy, and Success
Published
3 months agoon
February 24, 2025By
Brand Buzz
Jaipur, India – February 24, 2025 – In the ever-evolving digital world, staying ahead requires more than just theoretical knowledge—it demands hands-on expertise, strategic execution, and real-world application. Recognizing this need, DigiRoads has emerged as a leading force in digital marketing education, offering cutting-edge training programs designed to equip learners with industry-driven skills. Whether you are an aspiring marketer, a business professional, or an entrepreneur, DigiRoads empowers individuals to master digital marketing strategies that drive tangible success.
DigiRoads: Where Learning Meets Real-World Implementation
With the rapid expansion of the digital economy, the demand for skilled digital marketers has skyrocketed. Companies are constantly looking for professionals who can optimize campaigns, analyze data, and execute marketing strategies effectively. DigiRoads was founded with a mission to bridge the gap between conventional education and real-world application, ensuring students not only understand marketing concepts but also implement them through practical training and live projects.
From Humble Beginnings to a Digital Powerhouse
Laying the Foundation: Building Expertise & Delivering Results
DigiRoads was founded in 2015 by two visionary BITS Pilani alumni, who sought to revolutionize digital marketing education. The early years were focused on helping businesses enhance their digital presence through SEO, PPC, and social media marketing.
During this period, DigiRoads achieved:
- SEO Excellence: Ranked over 200+ websites on the first page of Google, boosting organic traffic for businesses across industries.
- PPC Mastery: Created high-performing ad campaigns that tripled ROI for startups and established enterprises.
- Social Media Growth: Launched viral marketing campaigns that increased audience engagement by 150%.
These achievements positioned DigiRoads as a trusted digital marketing partner for businesses seeking data-driven strategies and measurable growth.
The Rise of DigiRoads Classes
As the demand for skilled digital marketers surged, DigiRoads took a significant step in 2023 by launching DigiRoads Classes, a career-focused training institute designed to provide hands-on learning experiences. Unlike traditional training programs that focus only on theory, DigiRoads Classes ensures students actively implement strategies on live projects, preparing them for real-world marketing challenges. The institute offers specialized courses, including SEO Course, PPC Course, Social Media Marketing Course, Performance Marketing, Content Marketing, and Web Analytics. Each program is tailored to industry standards, equipping students with the most in-demand skills.
Since its inception, DigiRoads Classes has successfully trained over 500+ students, many of whom have secured high-paying jobs, internships, or started their own digital marketing agencies. The impact of the courses extends beyond professional growth—students experience enhanced critical thinking, analytical skills, and creative problem-solving abilities. The positive side effects include improved confidence, strategic mindset development, and the ability to adapt to fast-changing digital trends. With expert mentorship, industry-recognized certifications, and job placement assistance, DigiRoads Classes continues to be the top choice for digital marketing aspirants looking to excel in this competitive field.
Shaping Careers with Industry-Driven Projects
DigiRoads has transformed businesses while providing students with the best digital marketing course in Jaipur for real-world marketing exposure. Some of its most impactful projects include:
1. E-Commerce Brand Success
- Boosted website traffic by 400% within six months using SEO and targeted PPC campaigns.
- Increased online sales fivefold through performance-driven marketing strategies.
2. Real Estate Digital Expansion
- Designed a lead-generation strategy for a real estate firm, resulting in a 200% increase in customer inquiries.
- Leveraged Facebook & Google Ads to drive high-intent leads and improve retention rates.
3. Healthcare Digital Growth
- Developed an SEO-driven content strategy that ranked a healthcare startup in the top three search results for competitive keywords.
- Achieved a 60% increase in patient bookings through advanced digital advertising.
Key Achievements




Why Choose DigiRoads?
Unlike traditional training programs, DigiRoads focuses on real-world execution. Students don’t just study digital marketing—they apply it through live projects, hands-on mentorship, and performance-driven learning.
What Sets Us Apart?





DigiRoads is committed to producing skilled digital marketers who can excel in the competitive job market. Our structured digital marketing course in Jaipur ensures learners develop a strong foundation in SEO, PPC, content marketing, web analytics, and more.
Start Your Digital Marketing Journey Today!
Whether you are looking to start a career in digital marketing, upgrade your skills, or enhance your business’s online presence, DigiRoads offers the best digital marketing course in Jaipur tailored to industry demands.
At DigiRoads, we don’t just teach marketing—we help you implement strategies that drive real results. Join us and take the next step towards becoming a digital marketing expert.
Visit www.digiroadsclasses.in or email us at info@digiroadsclasses.in for inquiries. Call us at +91-8971129995.
Business
Flipkart Offers Huge ₹26,000 Discount on Google Pixel 9—Grab the Deal Now!
Published
3 months agoon
February 20, 2025Tech enthusiasts looking for a high-end smartphone upgrade can now grab the Google Pixel 9 at an unbelievable discount on Flipkart. The e-commerce giant is offering a massive price cut on the flagship Pixel 9, making it a great opportunity for buyers to own this premium device at a much lower price.
Google Pixel 9 Discount Details on Flipkart
The Google Pixel 9 was originally priced at ₹79,999, but Flipkart is now offering the device for ₹74,999, giving an instant discount of ₹5,000. However, additional offers can further lower the price by a significant margin.
Additional Bank Offers
Flipkart is providing additional discounts through select bank offers:
- HDFC Bank Credit Card EMI Transactions: Customers using HDFC Bank credit cards for EMI purchases can avail an extra ₹4,000 discount, bringing the effective price down to ₹70,999.
Exchange Offers: Get Up to ₹38,150 Off
One of the biggest attractions of this deal is Flipkart’s exchange offer, allowing users to trade in their old smartphones for an additional discount. Depending on the model and condition of the exchanged device, buyers can get a discount of up to ₹38,150. If the maximum exchange value is applied, the effective price of the Google Pixel 9 can drop to as low as ₹32,849.
Google Pixel 9 Specifications
For those considering the Pixel 9, here’s a quick look at its top features:
- Display: 6.3-inch OLED with high refresh rate
- Processor: Google Tensor G4 for enhanced performance
- RAM & Storage: 12GB RAM, 256GB internal storage
- Rear Camera: 50MP (main) + 48MP (ultrawide) dual-camera setup
- Front Camera: 10.5MP for high-quality selfies
- Battery: 4700mAh with fast charging support
- Software: Runs on the latest Android 15 with guaranteed updates for 7 years
The Pixel 9 is available in multiple color variants, including porcelain, obsidian, Peony, and wintergreen, giving users stylish options to choose from.
How to Avail This Offer on Flipkart?
To grab this deal, follow these steps:
- Visit Flipkart: Go to Flipkart’s official website or app.
- Select the Google Pixel 9—choose your preferred color and variant.
- Check for Bank Offers: Ensure you apply the HDFC Bank discount if eligible.
- Opt for Exchange (if applicable): If you have an old smartphone, select the exchange option and check the discount available for your device.
- Proceed to Payment: Complete the transaction and enjoy your new Google Pixel 9 at a heavily discounted price.
Is This the Right Time to Buy?
With a massive discount of up to ₹26,000, this Flipkart deal makes the Google Pixel 9 an irresistible buy for those wanting a premium smartphone with flagship-level performance, AI-powered cameras, and Google’s software experience. If you have been considering an upgrade, now is the perfect time to grab the Pixel 9 at its lowest price yet.
